Local News
Opvee, an additional opioid overdose reversal medication, helps in the fight against the opioid crisis
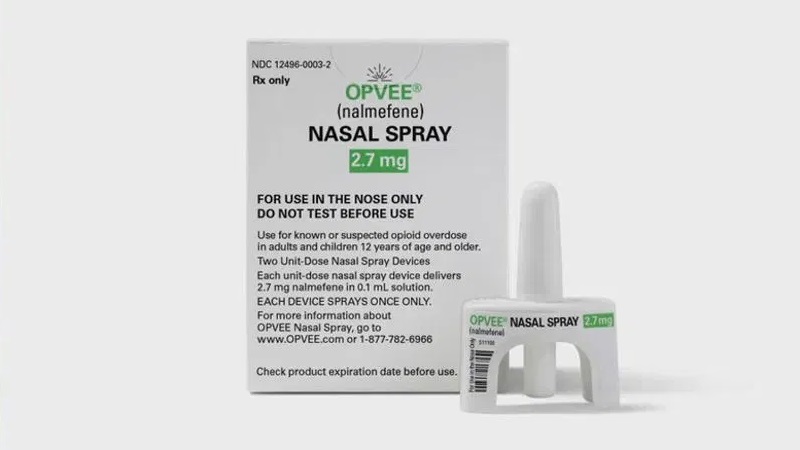
Little Rock, Arkansas – A new medication that can prolong the symptoms of an overdose is now available in Arkansas and has been gradually making its way throughout the nation to combat the opioid overdose issue.
The medication, known as Opvee, is the first nasal spray containing nalmefene to receive FDA approval; it was approved in May of 2023.
It is an additional measure in the battle against accidental and addiction-related overdoses.
Fentanyl overdoses in Arkansas increased by 251% between 2019 and 2021.
The Arkansas Opioid Dashboard reports that 487 overdoses in 2022 were fatal out of a total of 4,324 overdoses.
Judi Dane, Senior Director of Communications at Opvee, said the medication is comparable to Narcan in that it reverses the effects of an opioid overdose.
“This is the first time Nalmefene is available in a nasal spray,” said Dane. “Nalmefene had been approved in 1995 by the FDA so there is a long history of the effectiveness of Nalmefene in different formulations.”
It is simple to administer Opvee to someone who might be overdosing, just like Narcan.
According to Dane, it is accessible in 33 states nationwide in addition to Arkansas since the Arkansas Department of Health issued a standing order.
“When we look at the crisis we have today with over 100,000 people losing their life to overdose in the last 12 months, I think we can all agree that we need every tool available to be able to combat this and reverse those trends,” said Dane.
Opvee and Narcan are slightly different, according to Anne Pace of Kavanaugh Pharmacy, despite their similarities.
Regardless of which medication is used—Narcotic will wear off in a few minutes while Opvee may take several hours—the patient needs to travel to the hospital right once.
“You can go back into opioid overdose,” said Pace. “Opvee is longer acting and will stay in the body and on those receptors longer. This is a big deal because fentanyl lasts a lot longer.”
According to Pace, these medications represent a significant advancement in the fight against the opioid problem.
“It is a huge problem,” said Pace. “The more available we can make these drugs, so if this drug is better and better for some specific people, as much as we can get out there, I think that is a win.”
There’s another thing Opvee needs to be mindful about, Pace cautioned.
Opvee has a longer half-life on your receptors, therefore there’s a chance the patient may experience withdrawal symptoms right away.
To help with those symptoms, they must get medical help as soon as possible. Following FDA clearance, Opvee was added to the Arkansas Opioid Antagonist Protocol, according to the Executive Director of the Arkansas State Board of Pharmacy: “This drug was added as an additional opioid antagonist option with FDA approval and was subsequently added to the protocol. Arkansas Statutes speak to opioid antagonists which were previously seen as only naloxone products as a rapid acting opioid reversal agent until this new product came out. Nalmefene is a longer acting reversal agent than Naloxone. With some Naloxone use we hear of situations where the naloxone wears off and the person goes back into overdose thus the need for further clinical oversight by medical professionals and possible additional doses of naloxone in these situations. Overall, Naloxone is the preferred overdose reversal product but Nalmefene is a newer, longer acting product that is also available.”
-

 Local News5 days ago
Local News5 days agoFormer publisher of National Enquirer reveals 2018 hush money discussions with Governor Sanders
-

 Local News2 weeks ago
Local News2 weeks agoApplications for the fifth cohort of the Women’s Economic Mobility Hub are being accepted
-

 Local News2 weeks ago
Local News2 weeks agoSen. Cotton: During the Malinowski raid, ATF officers were not wearing body cameras
-

 Local News2 weeks ago
Local News2 weeks agoArkansas State Capitol passes resolutions on cryptocurrencies
-

 Local News2 weeks ago
Local News2 weeks agoDavid Pryor, an 89-year-old former senator and governor of Arkansas, passed away
-

 Local News1 week ago
Local News1 week agoAn Arkansas native uses children’s literature to embrace uniqueness and encourage dreams
-

 Local News2 weeks ago
Local News2 weeks agoSen. Cotton: During the Malinowski raid, ATF officers were not wearing body cameras
-

 Local News1 week ago
Local News1 week agoASP looks for a suspect in the teen’s death following the Helena-West Helena prom






Leave a Reply